
Starting in the cosmetic packaging industry can be challenging. Knowing what documents and materials you need can save time and ensure compliance. Whether you’re designing packaging or preparing for production, the right preparation is critical.
To succeed in the cosmetic packaging industry, you need proper materials like glass1, plastic2, or paper, and documentation such as FDA-compliant labels3, ISO certifications4, and product specifications.
Let’s explore these requirements in detail to help you get started.
What material is used for cosmetic packaging?
Choosing the right material is vital for protecting your product and ensuring its appeal.
Common materials for cosmetic packaging include glass, plastic, aluminum, and paperboard, depending on product type and sustainability goals.

Popular Materials:
- Glass: Used for perfumes and serums, known for its premium look and recyclability.
- Plastic: Lightweight, durable, and versatile for products like lotions or creams.
- Aluminum: Ideal for sprays or compact containers due to its resistance to corrosion.
- Paperboard: Often used for outer packaging like boxes, offering eco-friendly options.
| Material | Characteristics | Common Uses |
|---|---|---|
| Glass | Recyclable, luxurious | Perfumes, serums |
| Plastic | Lightweight, durable | Lotions, creams |
| Aluminum | Corrosion-resistant | Aerosols, compact cases |
| Paperboard | Eco-friendly, customizable | Outer boxes, cartons |
What are the FDA requirements for product labeling?
Proper labeling is crucial for compliance and consumer trust in the U.S. market.
The FDA requires cosmetic labels to include product identity5, net quantity6, manufacturer details, ingredient list7, and warning statements.

Key Label Elements:
- Product Identity: Clear identification of the product’s purpose.
- Net Quantity: Exact weight or volume of the product.
- Manufacturer Details: Name and address of the company.
- Ingredient List: All ingredients listed in descending order of predominance.
- Warnings: Any applicable usage warnings for consumer safety.
| Label Component | Example | Purpose |
|---|---|---|
| Product Identity | "Moisturizing Cream" | Informs customers |
| Net Quantity | "50 ml (1.7 fl oz)" | Ensures accuracy |
| Manufacturer Details | "XYZ Cosmetics, NY" | Builds accountability |
| Ingredient List | "Water, Glycerin, etc." | Ensures transparency |
| Warning Statements | "Avoid eye contact" | Promotes safe usage |
What is the ISO standard for the cosmetic industry?
Global standards ensure quality, safety, and consistency in the cosmetic packaging industry.
ISO 22716:20078 is the international standard for cosmetic Good Manufacturing Practices (GMP). It focuses on quality control, hygiene, and documentation throughout the production process.
ISO 22716 Highlights:
- Hygiene Standards: Maintains cleanliness in facilities and personnel.
- Documentation: Tracks processes for consistency and traceability.
- Quality Control: Ensures products meet defined safety and quality criteria.
| ISO Requirement | Description | Benefit |
|---|---|---|
| Hygiene | Clean facilities and staff | Reduces contamination risk |
| Documentation | Process tracking | Improves accountability |
| Quality Control | Safety and quality checks | Ensures compliance |
What information must appear on a cosmetic product?
Labeling is not just a regulatory requirement—it’s also a communication tool for customers.
Every cosmetic product must display product identity, ingredient list, net quantity, usage instructions, and manufacturer or distributor details.
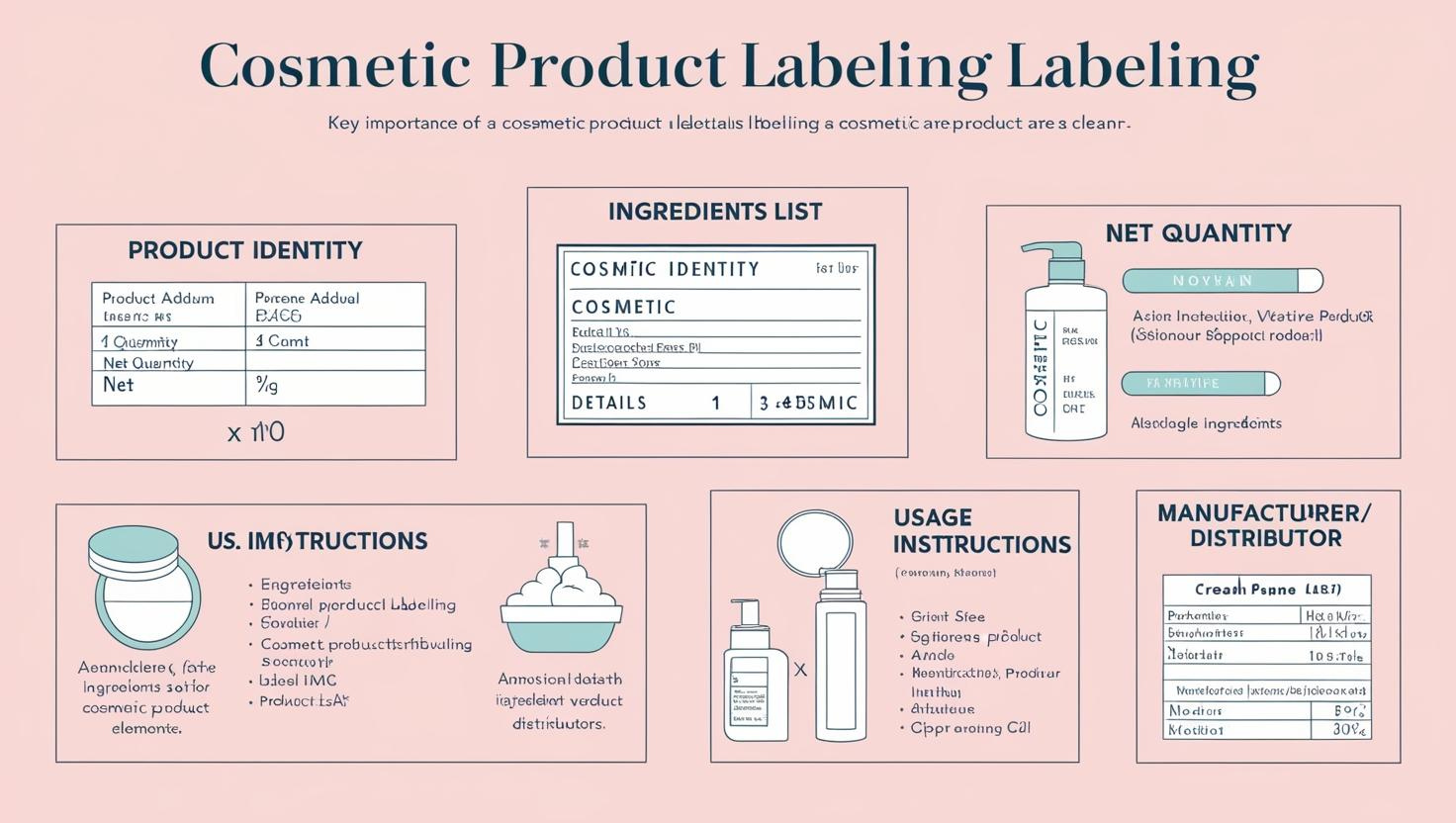
Must-Have Label Information:
- Identity: Clearly describes the product (e.g., “Lip Balm”).
- Ingredients: Transparency in the formulation.
- Net Quantity: Helps consumers compare products.
- Usage Instructions: Guides proper use to prevent misuse.
- Manufacturer Details: Establishes authenticity and trust.
| Label Element | Example | Importance |
|---|---|---|
| Identity | "Hydrating Face Mist" | Clarifies product type |
| Ingredients | "Aloe Vera, Vitamin E" | Builds transparency |
| Net Quantity | "100 ml (3.4 fl oz)" | Ensures accuracy |
| Usage Instructions | "Spray 2–3 times daily" | Improves user experience |
| Manufacturer Details | "ABC Beauty Co., USA" | Ensures accountability |
Conclusion
Entering the cosmetic packaging industry requires careful preparation. From selecting materials to meeting regulatory requirements like FDA labels and ISO standards, every step ensures product safety, compliance, and appeal.
-
Discover the benefits of using glass for cosmetic packaging. ↩
-
Learn why plastic is a popular material for cosmetic containers. ↩
-
Understand how to create FDA-compliant labels for cosmetics. ↩
-
Explore the role of ISO certifications in the cosmetic industry. ↩
-
Find details about product identity labeling requirements for cosmetics. ↩
-
Learn how to calculate and display net quantity on cosmetic labels. ↩
-
Discover the FDA requirements for ingredient lists in cosmetics. ↩
-
Review the key points of ISO 22716:2007 for cosmetic GMP standards. ↩


